Efficacy and Safety
Efficacy Data
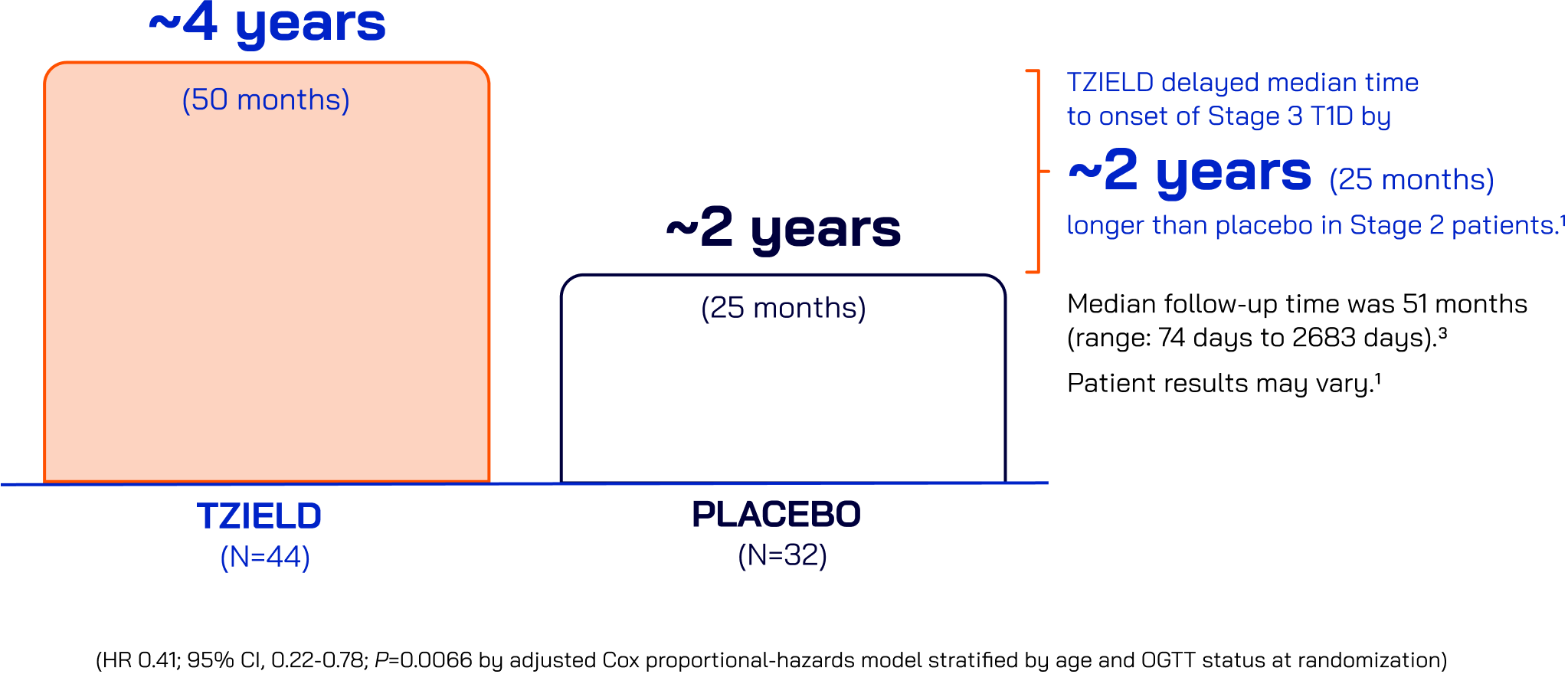
In the TN-10 Study with patients with Stage 2 T1D
One 2-week course of TZIELD led to twice the median time to
Stage 3 onset vs placebo1,2
Stage 3 onset vs placebo1,2
Median time without progression to Stage 3 T1D
At the time of the TN-10 extension analysis (~8.5 years after first patient start)†
29 of the original 76 study participants with Stage 2 T1D had not
progressed to Stage 33
progressed to Stage 33
Time without progression to Stage 3 T1D (in years)
50% of TZIELD-treated patients (22/44) with Stage 2 T1D had not progressed to Stage 3 at the end of the extended follow-up analysis versus 22% of placebo-treated patients (7/32)


Extended follow-up limitations
These data are not contained in the Prescribing Information. The TN-10 study was relatively small at the start of the trial, and patient numbers decreased throughout follow-up. Therefore, definitive conclusions cannot be derived from these data. Patient results may vary.3
†Median time of 923 days (~2.5 years; range, 74 days to 3119 days; ~0.2 to ~8.6 years).
At the end of the extended follow-up analysis,
10 of 13 subjects followed for
5 years were not diagnosed with T1D. These represent 18% (8/44) of the teplizumab group and 6% (2/32) of the placebo group of the original participants.3
Safety data
TZIELD is an immunomodulator with a well-established safety profile*
Common adverse reactions (ARs) in the TN-10 study1†‡
| Adverse Reactions | Placebo (N=32) | TZIELD (N=44) |
|---|---|---|
| Lymphopenia | 6% | 73% |
| Rash§ | 0% | 36% |
| Leukopenia | 0% | 21% |
| Headache | 6% | 36% |
| Neutropenia | 3% | 11% |
| Increased alanine aminotransferase | 3% | 5% |
| Nausea | 3% | 5% |
| Diarrhea | 0% | 5% |
| Nasopharyngitis | 0% | 5% |
Throughout the study, greater incidences of these ARs were reported in TZIELD-treated patients vs placebo-treated patients:
- Cytokine release syndrome (2% vs 0%)
- Serious infections|| (9% vs 0%)
- Hypersensitivity reactions and serum sickness (2% vs 0%)
- Lymphopenia (73% vs 6%)
- Neutropenia (7% vs 3%)
The approval of TZIELD was supported by a pooled safety analysis spanning 5 clinical trials including 773 patients1¶
*Adverse reactions in TZIELD-treated patients were also evaluated in a larger pooled safety analysis of adult and pediatric patients (773 received TZIELD and 245 received placebo or standard of care) who participated in 5 controlled clinical studies (including Study TN-10).
†ARs that occurred in 2 or more TZIELD-treated patients.
‡That occurred during treatment and through 28 days after the last study drug administration.
§Composite of rash-related terms, including rash erythematous, rash macular, rash papular, rash maculo-papular, rash pruritic.
||Serious infections included cellulitis, gastroenteritis, pneumonia, and wound infection any time during or after the first dose of study treatment.
¶These patients were studied using different dosages and time points: 1 study (TN-10) in patients with Stage 2 T1D, 3 placebo-controlled studies in an unapproved population, and 1 open-label standard-of-care controlled study of TZIELD in an unapproved population.
Cytokine release syndrome (CRS)
Of the 76 patients in the TN-10 study, CRS occurred in a single TZIELD-treated patient1
CRS can be mitigated by premedicating with antipyretics, antihistamines, and/or antiemetics, or by pausing dosing.
Premedicate, monitor liver enzymes, discontinue in those that develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal, and if severe CRS develops, consider temporarily pausing dosing.
Premedicate, monitor liver enzymes, discontinue in those that develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal, and if severe CRS develops, consider temporarily pausing dosing.
CRS manifestations in TZIELD-treated patients included:
- Fever
- Nausea
- Fatigue
- Headache
- Myalgia
- Arthralgia
- Increased alanine aminotransferase (ALT)
- Increased aspartate aminotransferase (AST)
- Increased total bilirubin
Most patients who received TZIELD experienced mild lymphopenia, a common adverse event that often resolved by Week 62
Lymphocyte count began to recover after Day 5 and returned to baseline by Week 6 in most patients.
Monitor white blood cell counts during the 2-week treatment period. If prolonged severe lymphopenia
(<500 cells per mcL lasting 1 week or longer) develops, discontinue TZIELD.
(<500 cells per mcL lasting 1 week or longer) develops, discontinue TZIELD.
Average lymphocyte count during TN-10 study1,2
Average absolute lymphocyte counts reached a nadir by Day 5 and then began to recover and returned to baseline by Week 6 in most patients


Adapted from Herold KC, et al. Means and confidence intervals are shown.
TZIELD has no black box warning, contraindications or known drug-drug interactions.1
ADDITIONAL WARNINGS
Additional warnings and precautions
Serious infections
Use of TZIELD is not recommended in patients with active serious infection or chronic infection. Monitor for signs and symptoms of infection during and after TZIELD treatment. If a serious infection develops, discontinue TZIELD.
Vaccinations
Administer all age-appropriate vaccinations prior to starting TZIELD. See recommendations regarding live-attenuated, inactivated, and mRNA vaccines.
Hypersensitivity reactions
If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly. Acute hypersensitivity reactions, including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm, occurred in TZIELD-treated patients.
See WARNINGS AND PRECAUTIONS (section 5.0) in the Prescribing Information to learn more.
OGTT=oral glucose tolerance test; T1D=type 1 diabetes.
References: 1. TZIELD Prescribing Information. Provention Bio, Inc; 2023.