Imagine the possibilities of delaying Stage 3 T1D
TZIELD delayed the median time to the diagnosis of Stage 3 type 1 diabetes (T1D) in the TN-10 trial.1
Actor portrayal


In patients with Stage 2 T1D, TZIELD significantly delayed the median time to Stage 3 T1D onset compared with placebo.1
MEDIAN TIME TO STAGE 3 T1D
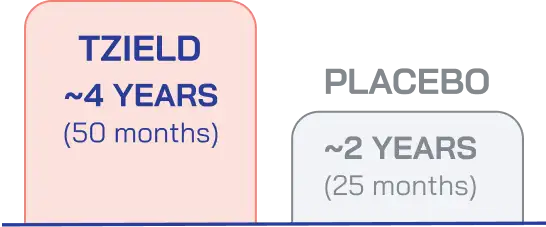
(HR 0.41; 95% CI, 0.22-0.78; p=0.0066 by adjusted Cox proportional-hazards model)
TZIELD delayed median time to onset of Stage 3 T1D by 25 months longer than placebo in Stage 2 patients. Median follow-up time was 51 months (range: 74 days to 2683 days).1,3
In the TN-10 trial extended follow-up, participants’ median time to Stage 3 T1D diagnosis was4:
MEDIAN TIME TO STAGE 3 T1D

(HR 0.457; p=0.01 by adjusted Cox proportional-hazards model)
In the extended follow-up of TZIELD- and placebo-treated patients of the TN-10 trial, the median follow-up time was 923 days (range, 74 days to 3119 days).4‡
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide.
View Important Safety Information page.